Obesity is a common metabolic disease that significantly increases the risk for type 2 diabetes, non-alcoholic fatty liver disease and cardiovascular disease. At its simplest, obesity is characterized by an accumulation of fat in the body stemming from an imbalance between caloric intake and energy expenditure. However, in reality, obesity is far more complex and is influenced by a myriad of genetic and environmental factors that impact signalling pathways in multiple tissues in the body. A central issue associated with obesity and its downstream complications is a dysfunction in lipid metabolism, reflected at both the whole-body level as well as in key metabolic tissues such as adipose tissue, liver, and skeletal muscle.
The Mutch Lab is focused on understanding how genetic and lifestyle factors impact lipid metabolism in models of health and metabolic disease. We are particularly interested in plant and marine omega-3 fatty acids due to their widely recognized health benefits through the regulation of lipid metabolism and inflammatory signalling pathways. Ongoing research in the Mutch Lab uses cell and rodent models to explore how altered dietary fatty acids influence cellular processes such as adipogenesis, lipogenesis, and lipolysis. Notably, our research has important implications for humans, since we and others have shown that humans with particular genetic variants show many of the same metabolic outcomes as our model systems.
Current Projects
Representative Publications:
Hatherell et al., Lipids, 2023
LeMoire et al., Prostaglandins Leukot Essent Fatty Acids, 2022
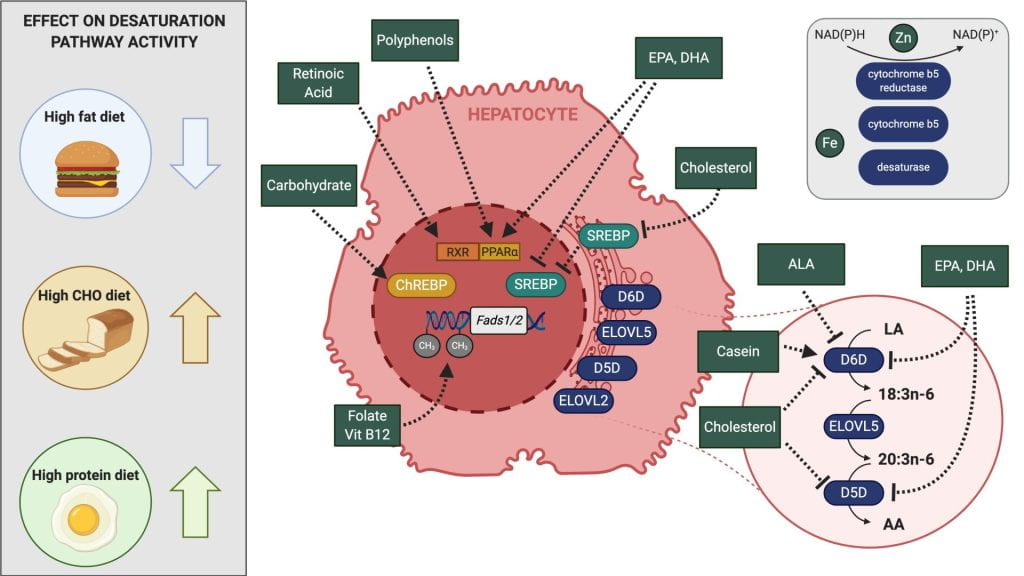
Diet and genetic regulation of omega-3 synthesis.
The essential omega-3 fatty acid, alpha-linolenic acid, is converted into eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in a pathway mediated by the delta-6 desaturase enzyme, which is encoded by the fatty acid desaturase 2 (FADS2) gene. EPA and DHA are important fatty acids that are generally obtained by consuming fatty fish. However, most people don’t consume fatty fish on a regular basis, so the synthesis of EPA and DHA through the desaturase pathway is of critical importance. We explore how macronutrients and micronutrients may regulate FADS2 gene expression and desaturase activity in both rodents and humans. We also explore how genetic variants in FADS2 alter desaturase pathway activity, either alone or in conjunction with various nutrients, and how this impacts common disease risk factors.
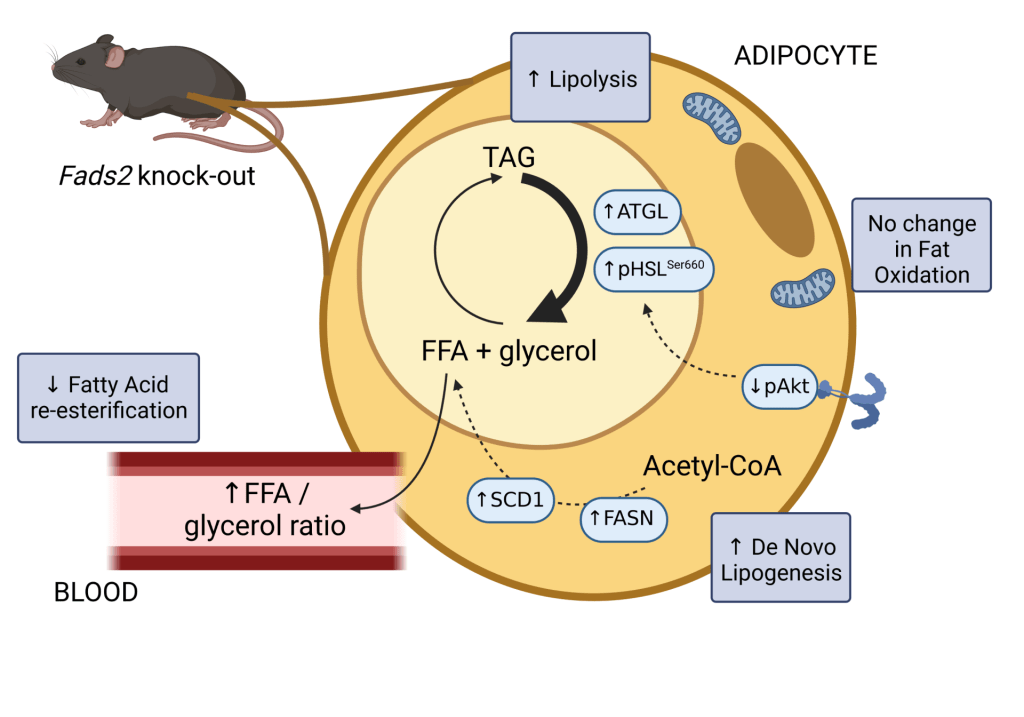
Omega-3 fatty acids and TAG/fatty acid cycling in adipose tissue.
Adipose tissue serves as a reservoir for the storage of excess energy in the form of triacylglycerol (TAG). In times of need, TAG undergoes lipolysis and is broken down into fatty acids and glycerol. The regulation of TAG/fatty acid cycling is critical for metabolic health and becomes dysfunctional with obesity and insulin resistance. We have discovered that the delta-6 desaturase enzyme, which plays an important role in omega-3 fatty acid synthesis, influences this cycle in adipose tissue. Using adipocyte cell models and the Fads2-/- mouse, we are exploring how omega-3 fatty acids regulate adipose tissue lipid metabolism in different contexts (e.g., diet-induced obesity, insulin resistance, etc).
Representative Publications:
Representative Publications:
MacIntyre et al. Metabolites, 2023
Precision Nutrition for Metabolic Health.
Precision nutrition is an emerging field that evaluates genetics, dietary patterns, the microbiome, and other lifestyle factors to determine the most effective plan to help prevent and alleviate disease in an individual. A primary objective of our research is to translate new knowledge regarding the diet and genetic regulation of lipid metabolism to humans. Not everyone responds in the same way to the same lifestyle intervention, with some people experiencing a benefit, others experiencing no change, and others experiencing an adverse outcome. Using genomic and metabolomic technologies, we are studying the basis for these different responses between people as well as their consequences on metabolic health.


